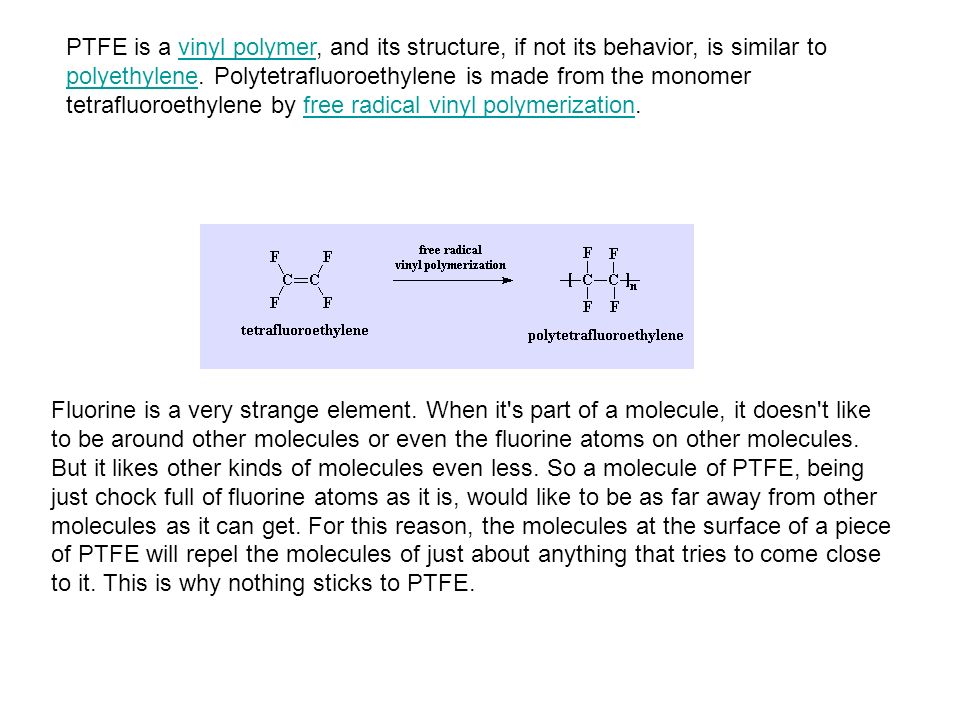
Teflon is produced from free radical vinyl polymerization of tetraflouroethylene resulting in the formation of polytetraflouroethylene or teflon.
Free radical vinyl polymerization of teflon.
Chemical characteristics of teflon chemically teflon belongs to the class of paraffin polymers.
Tetrafluoroethylene monomer nc f 2 c f 2.
The same steps are followed in the polymerization of other substituted alkenes.
Free radical polymerization last updated save as pdf page id 35129 radical chain growth polymerization contributors all the monomers from which addition polymers are made are alkenes or functionally substituted alkenes.
The combination of mofs with the raft technique enables the synthesis of highly isotactic poly vinyl ester s with a controlled molecular weight and narrow molecular weight distribution and stereocontrolled isotactic block atactic vinyl ester block copolymers.
Terylene melamine polymer and nylon 6 6 are synthesized using condensation step growth polymerization technique.
A linking small molecules together a type of addition.
Teflon is synthesized using a free radical addition or chain growth polymerization technique.
Free radical polymerization frp is a method of polymerization by which a polymer forms by the successive addition of free radical building blocks.
The plastics that have so changed society and the natural and synthetic fibres used in clothing are polymers.
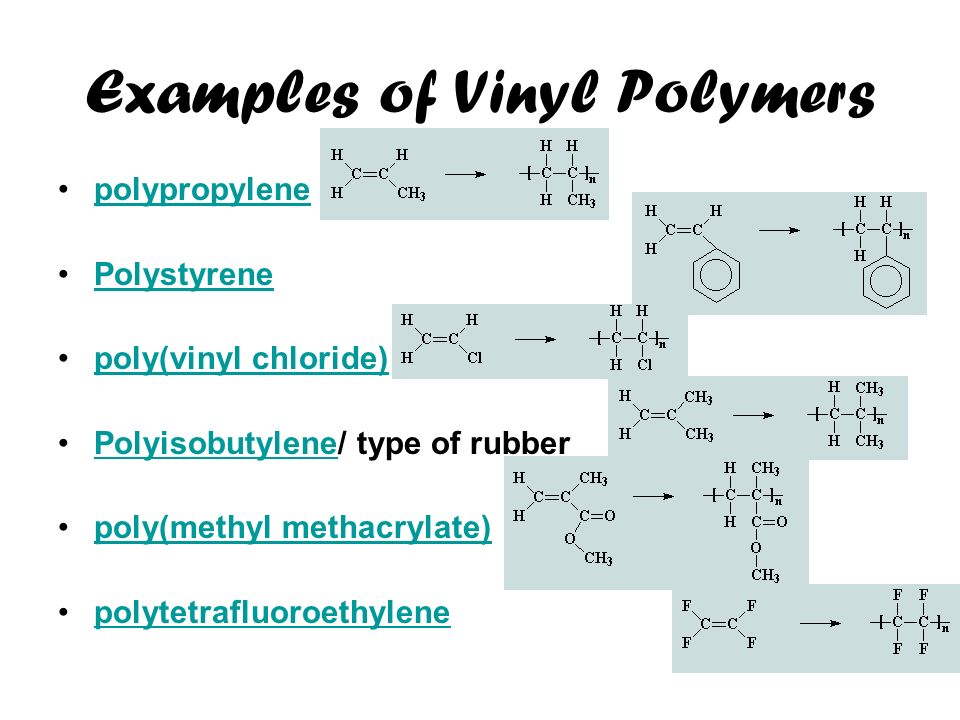
Simple alkenes are the source of many common plastics.
Most commonly addition polymerization is governed by free radical formation.
In table 3 1 the names structures and products formed in some polymerization reactions are listed.
Addition polymers namely polythene teflon and polyacrylonitrile are formed by a free radical mechanism of addition polymerization.
Free radical polymerization can be iniitated by thermal chemical or photolytic decomposition of initiator molecules.
R m rm.
Following its generation the free radical then reacts with a vinyl monomer that is it adds to one of the electrons of the double bond of the vinyl monomer and the remaining electron becomes the new free radical.
Steps in the free radical polymerization of vinyl chloride.
There are two basic ways to form polymers.
Free radicals can be formed by a number of different mechanisms usually involving separate initiator molecules.
Polymers are high molecular weight compounds fashioned by the aggregation of many smaller molecules called monomers.
However in this paraffin fluorine atoms replace all the hydrogen atoms.
Free radical and raft polymerization of vinyl esters with different molecular dimensions are conducted in the nanochannels of metal organic frameworks mofs.